Overview
Understanding the underlying mechanisms of gene interactions, uncovering druggable genes and modulation of regulatory pathways can lead to innovative treatment and preventative plans for families affected or at high risk of having a child with craniofacial birth defects.
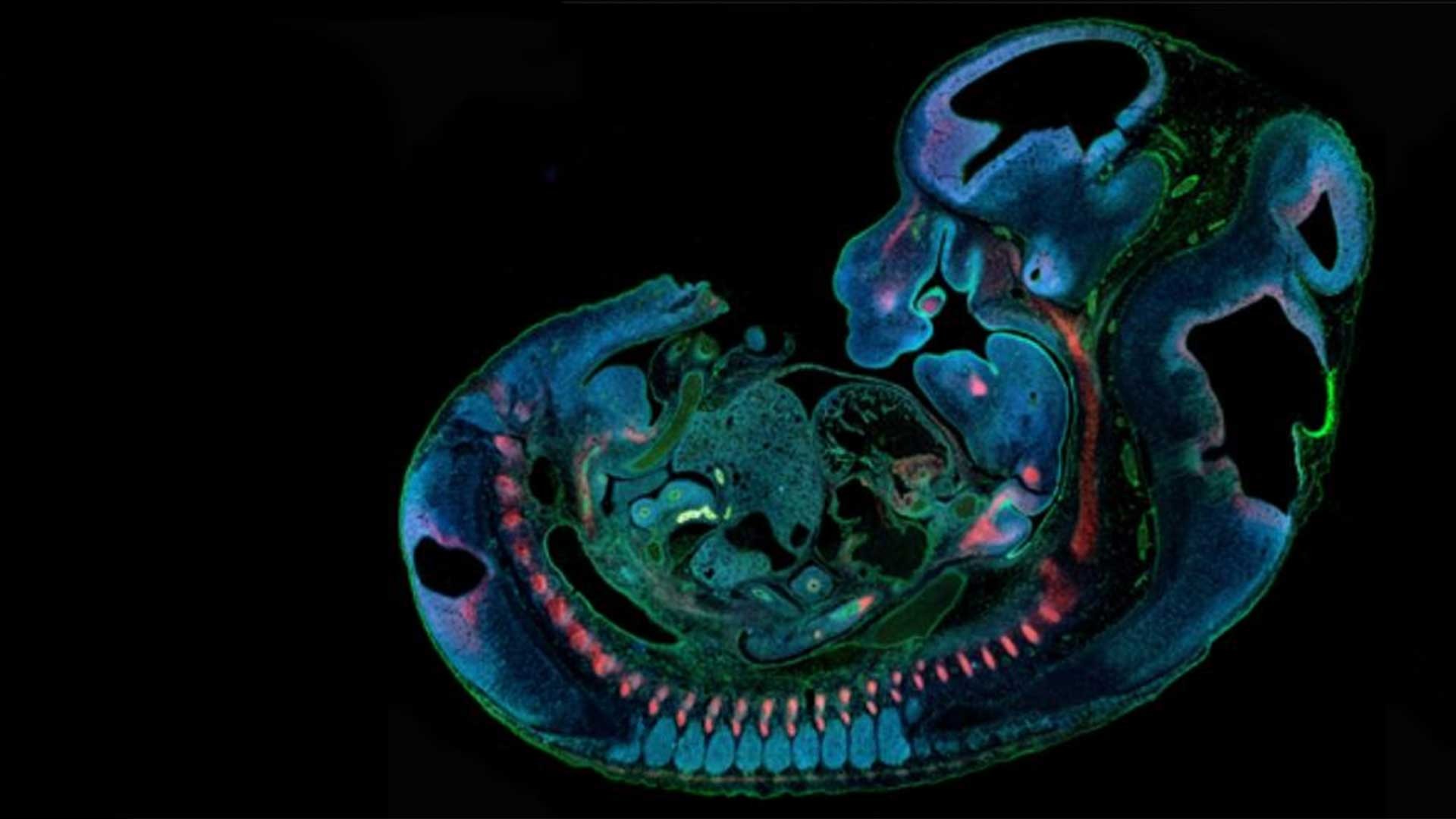
Our lab utilizes mouse models and organ cultures to delineate the molecular mechanism of a novel genetic interaction between two transcription factors, IRF6 and TWIST1, that plays a critical role in regulating the epithelial-mesenchymal interaction during oral, facial and skull development. By applying both biochemical and genetic approaches, the lab investigates how mutations in TWIST1 phospho-sites disrupt formation of craniofacial tissues derived from mesenchymal cells. The lab also integrates experimental data and bioinformatics for developing computational models to identify etiologic non-coding DNA variants associated with cancer diseases, including head and neck squamous cell carcinoma.
The goal of our research is to translate bench findings into clinical use to improve the risk assessment, healthcare and overall well-being of families affected with craniofacial birth defects by advancing personalized medicine.
-
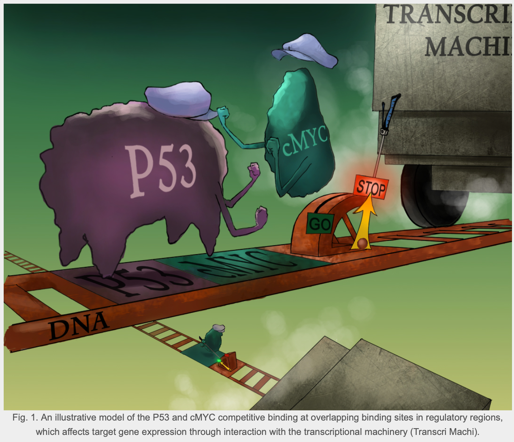
A Computational Model for Predicting Deleterious Non-coding DNA Variations in Cancer
Identification of causative DNA variants in common complex diseases is very important to screen individuals with high-risk for diseases and for developing therapies to target the genetic cause of diseases, in particular cancer. DNA variations that lie outside the protein-coding regions can alter gene expression without affecting its protein function. This research area is critical in the context of disease susceptibility and severity. Basic and translational research has been previously directed towards DNA variations located within sequences that code for protein products due to their obvious effect on the function of the corresponding protein. However, recent genome-wide association studies demonstrate that the majority of DNA variants associated with cancer and other common complex diseases are located in outside the protein coding-regions, while few causative regulatory variants have been identified so far. The specific function of sequences that lie outside the protein-coding regions is not known for the majority of the loci compared to those within the coding regions, which makes prediction of the effect of non-coding variants challenging.
Funding
R15, NIH-GH122030-01
-
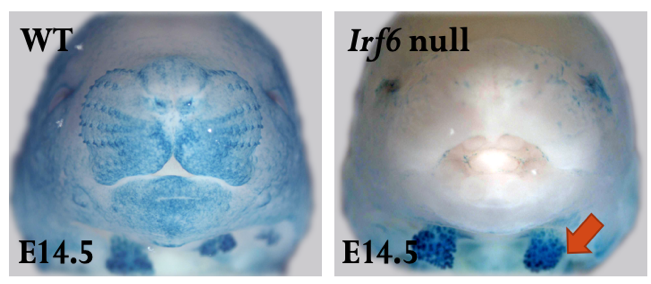
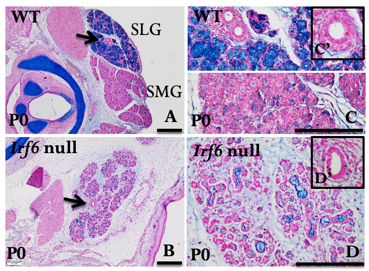
A novel function of IRF6 in salivary gland development
Xerostomia (dry mouth) is a common health problem that can cause long-lasting harm to affected individuals and substantially decreases their quality of life. Xerostomia occurs due to reduced salivary flow (hyposalivation) or changes in saliva composition as a result of damaging environmental or genetic factors that affect salivary gland (SG) function, such as autoimmune disorders. Hyposalivation is the most common complication of radiation therapy for head and neck cancer. Prevention of hyposalivation due to SG dysfunction or autoimmune disorders is currently unavailable. Therefore, there is a dire need to develop innovative therapies to prevent and treat this health issue. Human genetic studies showed that haploinsufficiency of the transcription factor Interferon Regulatory Factor 6 (IRF6) causes Van der Woude syndrome associated with chronic inflammation of minor salivary glands. Our studies showed that IRF6 is necessary for SG development. Lack of Irf6 causes disruption in branching morphogenesis and acinar differentiation. Our analyses of RNA-seq, RTqPCR, and immunostaining identified the differentially altered genes in Irf6 null SGs, involved in TGFb3 pathway and immune system. We will investigate the Irf6-dependent pathway involved in acinar cell differentiation, and elucidate the function of Irf6 in SG maturation and inflammation in adult mice. We will determine if IRF6 binds to regulatory elements of Col9a2, Hoxb6, Erg1, and Ltbp4 and whether these genes can rescue the phenotype in Irf6 null explants. We will determine the role of IRF6 in SG maturation and cytokine-mediated inflammation in tissue-specific inducible Irf6 knockout mice. The findings of this proposal will lay the groundwork for future studies to determine the importance of the IRF6 pathway in SG differentiation and inflammation.
Funding
R03, NIH-DE027155-01 (Pending)
Publications
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6429578/
-
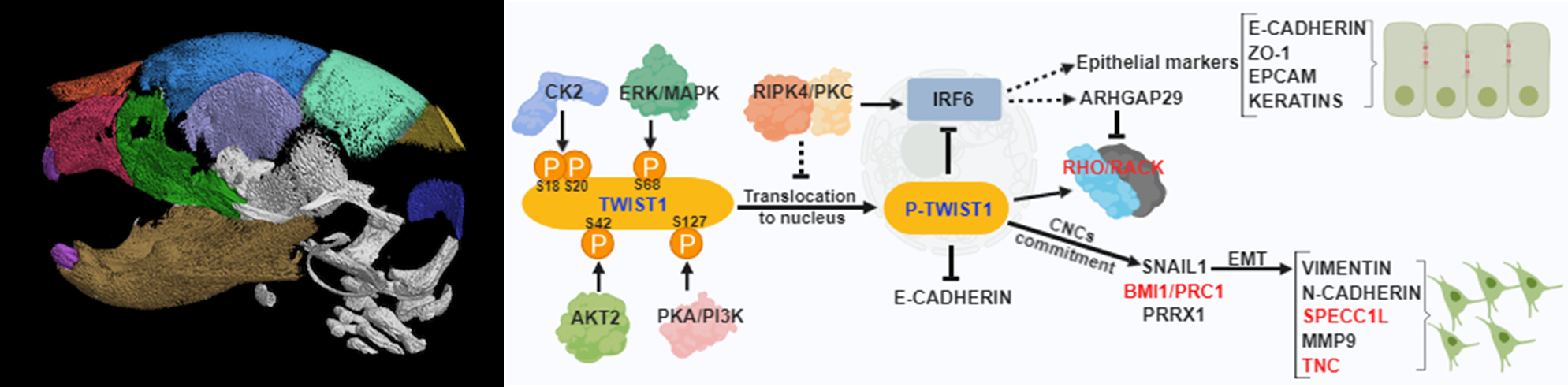
Regulation of cell fate during early craniofacial development
Fakhouri’s research focuses on understanding the genetic and epigenetic regulation of cell fate determination during neural tube and craniofacial development. His lab investigates the function of two developmental genes and their protein activities in regulating cell shape changes and cytoskeletal organization, and how the loss of function can lead to birth defects, including cleft lip and palate, craniosynostosis, and facial dysostosis. His lab uses mouse models, organ cultures, and biochemical assays to delineate further the underlying cellular mechanism and regulatory pathway that drive the proper formation of the neural tube and craniofacial bone development. His research aims to uncover druggable genes and signaling pathways that can be used to prevent and treat congenital birth defects.
Funding
R01, NIH/NIDCR (Pending)
-
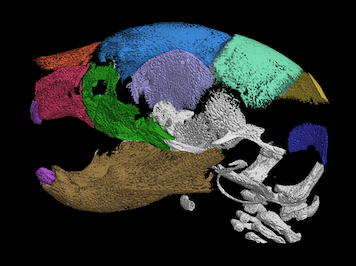
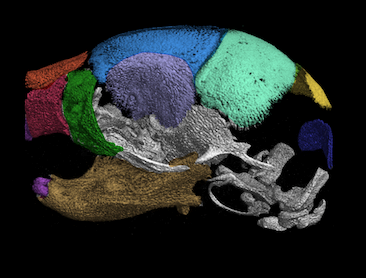
IRF6 in osteonecrosis of the jaw induce by bisphosphonates
A unique approach to uncover medication-related osteonecrosis of the jaw (MRONJ) is through genetic studies. Our lab has been investigating the transcription factor, IRF6, that functions as a tumor suppressor in bone development. Our published data has shown that Irf6 is expressed in cranial osteocytes, osteoclasts, and oral epithelium (Thompson, et al., 2019). The Irf6 gene is highly expressed in oral epithelium, mucosa, and macrophages, while moderately expressed in osteocytes. Loss of Irf6 leads to craniofacial bone abnormalities, including disorganized bone structure, reduction in the number of osteoclasts, and less mineralized bone. The bone matrix in Irf6 null mice is void of osteocytes in multiple regions of the mandible. Because the phenotype of Irf6 null mice shares bone matrix and cell profile characteristics with the histopathological presentation of MRONJ, it suggests that Irf6 might play a crucial role in the genetics of MRONJ.
Funding
AADR-Houston Chapter, UTHealth SOD
Publications
- Kin K, Bhogale S, Zhu L, Thomas D, Bertol J, Zheng WJ, Sinha S, Fakhouri WD. Sequence-to-expression approach to identify etiological non-coding DNA variations in P53 and cMYC-driven diseases. Hum Mol Genet. 2024 Sep 19;33(19):1697-1710. doi: 10.1093/hmg/ddae109. PMID: 39017605; PMCID: PMC11413647.
- Bertol JW, Johnston S, Ahmed R, Xie VK, Hubka KM, Cruz L, Nitschke L, Stetsiv M, Goering JP, Nistor P, Lowell S, Hoskens H, Claes P, Weinberg SM, Saadi I, Farach-Carson MC, Fakhouri WD. TWIST1 interacts with β/δ-catenins during neural tube development and regulates fate transition in cranial neural crest cells. Development. 2022 Aug 1;149(15):dev200068. doi: 10.1242/dev.200068. Epub 2022 Aug 8. PMID: 35781329; PMCID: PMC9440756.
- Zhang A, Aslam H, Sharma N, Warmflash A, Fakhouri WD. Conservation of Epithelial-to-Mesenchymal Transition Process in Neural Crest Cells and Metastatic Cancer. Cells Tissues Organs. 2021;210(3):151-172. doi: 10.1159/000516466. Epub 2021 Jul 2. PMID: 34218225; PMCID: PMC8387394.
- Adibi S, Seferovic D, Tribble GD, Alcorn JL, Fakhouri WD. Surfactant Protein A and Microbiome Composition in Patients With Atraumatic Intraoral Lesions. Front Oral Health. 2021 Apr 22;2:663483. doi: 10.3389/froh.2021.663483. PMID: 35048007; PMCID: PMC8757703.
- Metwalli KA, Do MA, Nguyen K, Mallick S, Kin K, Farokhnia N, Jun G, Fakhouri WD. Interferon Regulatory Factor 6 Is Necessary for Salivary Glands and Pancreas Development. J Dent Res. 2018 Feb;97(2):226-236. doi: 10.1177/0022034517729803. Epub 2017 Sep 12. PMID: 28898113; PMCID: PMC6429578.
View additional publications on NIH website
Announcements
- Drs. Fakhouri and Letra Special Issue Editors invited investigators to submit their research manuscripts to the Special Issue "DNA Variations in Evolution and Human Diseases"
- Drs. Fakhouri, Matsumoto, Biguetti, and Soldatos invite investigators tto submit their research manuscripts to the Special Issue "Craniofacial Bone and Dental Genetics, Metabolism, Aging, and Disorders"